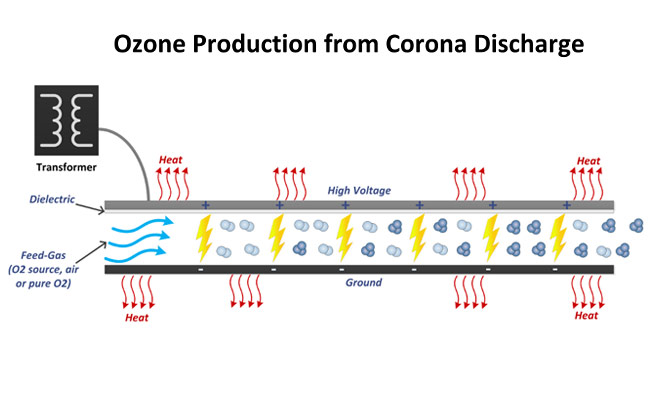
Ozone is being used as one of the best water disinfectants worldwide
Overview
Ozone is a natural gas, in which 3 atoms of oxygen are combined to form the molecule O3. It is an allotrope of oxygen having much less stability than the diomatic oxygen. Ozone was discovered by Schonbein in France in 1840. It has been named after the Greek word for smell (ozein). O3 is a active, colorless gas which has a distinct smell and is about one and half times heavier than air. Under pressure it becomes unstable and decomposes readily into molecular oxygen. The formation of O3 occurs with the use of energy from oxygen. The formation of Ozone takes place in upper atmosphere by U.V radiation. Commercially ozone is produced by passing the atmospheric air or oxygen into “Corona Discharge” produced by high electric field. Since, 1893 ozone is being used as one of the best water disinfectants worldwide
Advantages of Ozone
- Ozone is unstable and quickly decomposes to normal oxygen
- Ozone gas is slightly heavier than air
- Ozone is only partially soluble in water
- Ozone solubility decreases with a rise in temperature
- Ozone is a reactive substance
- Ozone is the most powerful of the commonly used drinking water oxidants
Ozone is widely used for drinking water treatment because of in excellent disinfection and oxidation qualities. Ozone is a strong, naturally occurring oxidizing and disinfecting agent and can be used safety water treatments. Ozone is 25 times more powerful than hypochlorite acid and 500 times faster. Ozone has greater disinfection effectiveness against microbes like bacteria, virus and cyst compared to chlorination. In addition, the oxidizing properties of ozone can also reduce the concentration of Iron, Manganese and reduce or eliminate the taste and odor problems in water. Thus, the characters of ozone make it the most superior in water disinfectants, comparing to other technologies.
- Bacterial Disinfection
- Viral and Cyst Inactivation
- Biofouling Control
- Color, Tastes & Odors
- Turbidity Control
- Selected Detergents
- Micro flocculation (of soluble organics)
- Selected Pesticides
- Pre-treatment of Organics for Biological Oxidation
- Trihalomethane (THM) Precursor Control
- Algae Control
- Phenols
- Ozone is an unstable molecule which quickly changes back to oxygen
- The time for half of the ozone in air to decompose is approx. 4 to 12 hours
- The half-life in water ranges between seconds and hours depending on temperature, pH and water quality
- Molecular ozone (O3)is most stable in high purity water when the pH is less than 6
- Ozone has a very strong oxidizing power with a short reaction time
- Ozone can eliminate a wide variety of inorganic, organic and micro biological problems, taste and odor problems
- Ozonation results in Safe and easy disinfection of water
- The maintenance cost of ozonation is low
- Ozone requires no additional disinfectant
- Since, Ozone is produced on-site using atmospheric air; it requires no storage of dangerous chemicals
- Iron
- Sulfides
- Manganese
- Nitrites
- Organically-bound Heavy Metals
- Arsenic
- Cyanides
- Computer generated model of a bacillus (rod shaped ) bacterial cell
- Ozone (green ) comes into contact with the cell wall. The cell wall is vital to the bacteria because it ensures the organisam can maintain its shape
- Once ozone makes contact, a mechanism called an oxidative burst occurs which literally creates a tiny hole in the cell wall
- A close- up view of the cell wall
- Image showing the cell after constant bombardment by the ozone
- After a few seconds, and thousands of ozone collisions later, the bacterial wall can no longer maintain its shape and the cell dies
Please leave a message for more details and we will get back to you soon.
Enquire Now
- Customer Care Services
+1800-103-1145
- Talk to Us